

You can measure the pH-value of a food using a pH-meter or a special pH-measurement strip. As such, lemon juice is more acidic than yogurt. For example, lemon juice has a pH-value of 2-3 whereas that of yogurt is around 4-4.5. The further away from that neutral value of 7 the more acidic (or alkaline) a food is. Foods with a pH lower than 7 are acidic, those with a pH higher than 7 are alkaline. Any food that has a pH value of 7 is neutral. In determining what a food is you use the pH-scale. Whether or not something is acidic or alkaline is very well defined by chemists. A food that is neither acidic or alkaline is called neutral. The opposite of an acid food (or drink) is one that is alkaline. If you drink any of these, you taste the acidity. Lemon & lime juice, vinegar, yogurt and buttermilk are all acid foods. When is a food acidic?Īcidic foods are pretty common. In this post we’ll discuss the underlying science of these reaction types. Acid/base reactions all have a lot in common, even though their effect (a colour change, leavened cake or ‘cooked’ fish) may look very different. These types of reactions are just one of many chemical reactions that happen in food (another one would be browning of bananas). They are all examples of acid/base reactions. What do all of these examples have in common?
You can ‘cook’ fish by adding lemon and lime juice, this makes a ceviche. Baking powder causes your cake to rise beautifully in the oven. Therefore, the name of the salt produced during this reaction is zinc chloride.Red cabbage juice changes colour when you add a squirt of lime juice or a spoonful of baking soda. This salt composed of zinc and chloride ions would be called zinc chloride. In order to have a neutrally charged compound, we need two chloride ions for every one zinc ion, giving us the formula ZnCl2.
Acid base reaction plus#
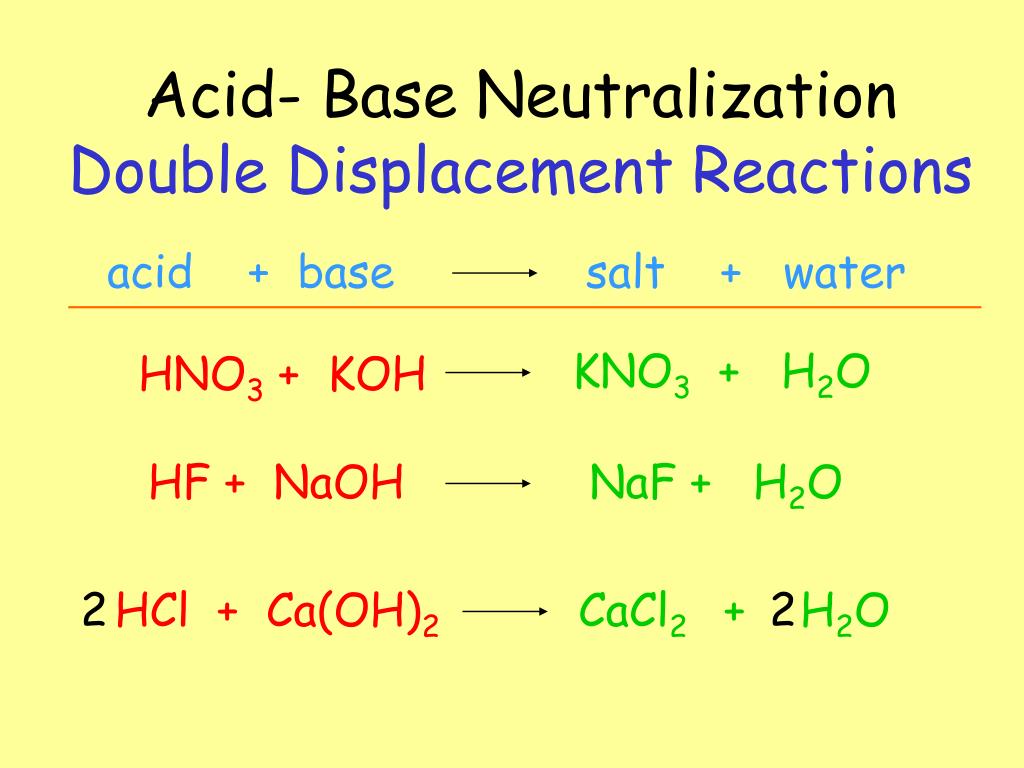
This compound is made of zinc two plus ions and chloride one minus ions. We know that the acid will contribute its anions, which are chloride ions. We know that the base zinc oxide will contribute its cations to the salt, which are zinc two plus cations.

To determine the name of the salt formed, let’s have a look at the ions it will be composed of. We know that one product of this acid–base reaction would be water. Using this pattern, let’s predict what salt would be formed from the reaction between zinc oxide and hydrochloric acid. So in an acid–base reaction, the salt product is formed from the cations of the base and the anions of the acid. The salt also contains bromide ions, which originated from the acidic reactant hydrobromic acid. This salt is made up of magnesium cations, which originated from the basic reactant magnesium oxide. The salt produced in this reaction is magnesium bromide. When the base magnesium oxide reacts with hydrobromic acid, magnesium bromide and water are formed. Let’s have a look at an example of an acid–base reaction. In this reaction, hydrochloric acid is reacting with the base zinc oxide. A few examples include acid–base reactions, reactions between acids and metals, and reactions between acids and carbonate compounds. Salts can be produced in many types of reactions with acids.

A salt is an ionic compound made of cations and anions. This question is asking us to identify the salt product from the reaction between zinc oxide and hydrochloric acid. What is the name of the salt produced during this reaction?


 0 kommentar(er)
0 kommentar(er)
